Neurosurgery and Oncology professor at Johns Hopkins, Dr. Gregory Riggins, along with his research group typically encounter little to no difficulty in enabling the growth of cancer in test mice for their studies.
However, during a span of several months back in 2009 there was one cluster of these mice where tumor development simply wouldn’t occur. Upon further examination they discovered that this unexpected outcome was due to these rodents having been administered fenbendazole as an anti-parasitic treatment.
“In our exploration of various medications within this particular group, fenbendazole emerged as the most effective. This gained our attention.” Said the researcher
As news began spreading about its abilities, individuals discovered that fenbendazole could be administered alone or combined with other procedures to cure or hinder cancer growth.
Riggins and Gallia along with their research squad initiated a scheme not only to comprehend how this medicine functions but also enhance its utility against glioblastoma cells and get it produced for patient trials. According to these researchers’ belief system, fenbendazole assists in blocking tumor development by restraining formation of tubulin strands – proteins vital for proliferation of cancerous cells.
This event occurred over a decade ago. Presently, with the passage of considerable time, we have gained substantial knowledge on employing fenben effectively to enhance our likelihood of eradicating cancer.
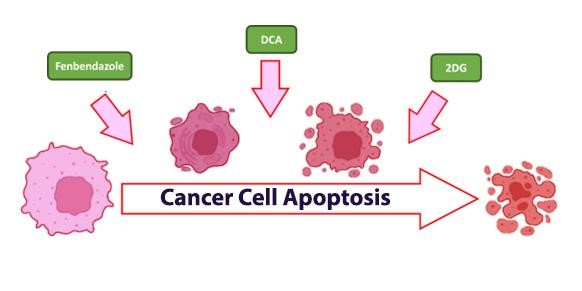
Revamped medications are making remarkable strides in cancer treatment. By understanding how cancer cells use energy, we see how compounds like Fenbendazole, DCA (Sodium dichloroacetate), and 2DG (2-deoxy-D-glucose) revolutionize standard treatments like chemo and radiation, offering new hope in cancer care.
Cancer’s Energy Structure
Back in 1923, Otto Heinrich Warburg – a pivotal German biochemist, practicing medical professional and Nobel Prize recipient – unveiled an invaluable discovery that played a major role in comprehending the energy metabolism pattern of cancer cells. This particular observed phenomena is currently acknowledged as the key characteristic of cancer dubbed more commonly referred to as “The Warburg Effect.”
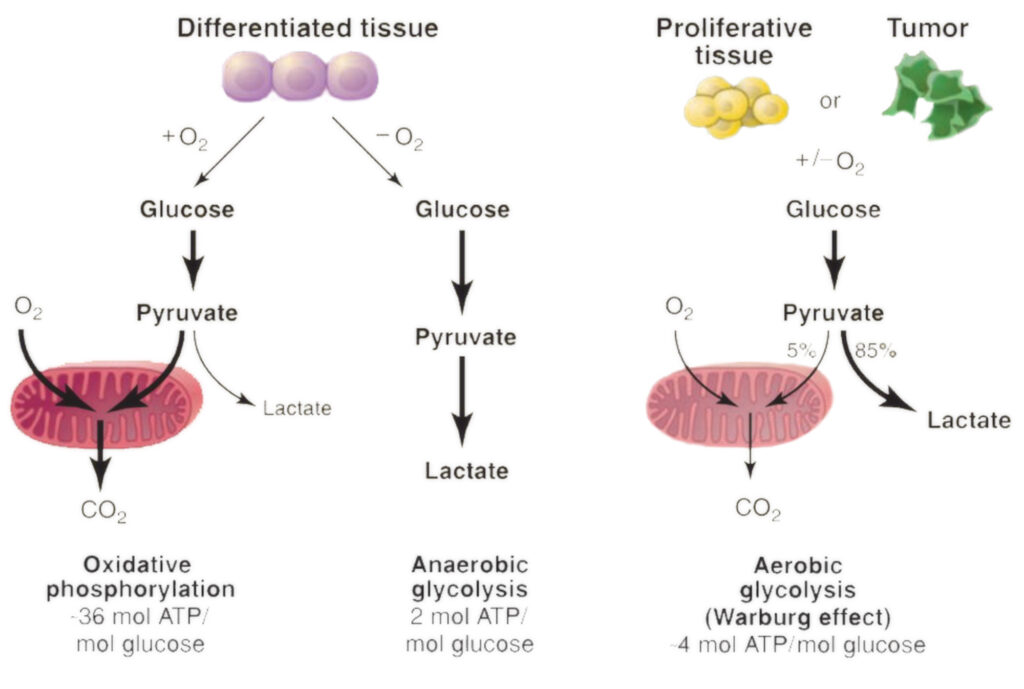
A notable observation made by Mr. Warburg was while studying rat tumor cells: he noted they flourished on exceptionally high levels of glucose (sugar) instead augmenting use oxygen for growth purposes which appeared counterintuitive given extracting energy from nutrients via higher utilization rates of oxygen would have been largely efficient process.
The establishment of such findings during those times around hundred years ago sparked substantial curiosity leading to unanswered questions than solutions available at hand.
The vast glucose craving and diminished oxygen consumption bestow on cancer cells a distinct set of evolutionary benefits. One manner in which the Warburg impact aids malignant cells is by facilitating swift accumulation of biomass. The uptake enhancement for glucose equips the tumor tissue with excess building blocks to create new genetic material and protein, thereby leading to an upsurge in both proliferation and expansion of cancer.
Additionally, it’s no secret that normal mammalian cells require a constant flow of oxygen or else they perish swiftly. However, their diseased counterparts behave entirely differently – growing tumors rapidly often outpaces their available O2 supply but does not slow down due to its adaptive nature; instead thriving under such circumstances without suffocating.
Shortly, the cancerous cells transition their metabolic process to oxygen-dependent glycolysis. They start generating and discharging abundant quantities of lactic acid beyond the cellular boundaries, consequently causing a rise in acidic conditions within tumor surroundings.
Increased acidity of such cancerous environments bolsters further penetration and spreading by degrading our body’s cell-connecting extracellular matrix. When this acid level escalates, it aids malignant growths in evading our immunity mechanism – an inherent guard against diseases like these. Undeniably one reason why immunotherapy often tends to lose its impact while working on progressed stages of cancer.
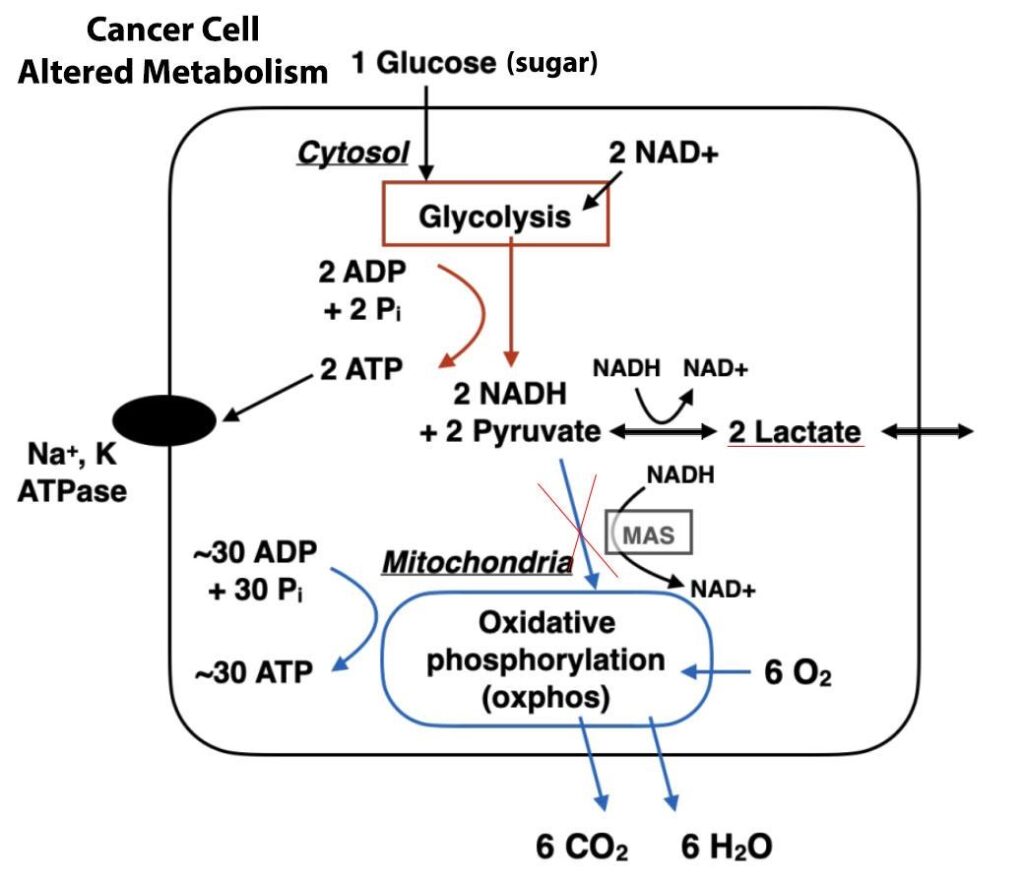
Simultaneously, cancer cells draw on less oxygen leading to the mitochondria within them generating fewer reactive oxygen species. These function crucially in both halting and deleting harmful cancerous cells. The disease starts dodging a mechanism termed as apoptosis.
Apoptosis is an ordinary phenomenon that comes into play in our body; it conveniently removes unwanted or excessive cells from continuing their existence with the anatomy.
It’s essentially natural pre-arranged cellular death stopping tumor growth at inception by exterminating itself primarily for preventing any potential unnecessary expansion of these tumors inside us causing damage beyond repair, thus rendering malignant cell bodies ceaseless.
These are key metabolic advantages enabling unregulated life cycle progression of various transforms while thriving indefinitely being immortal due to deterring programmed timely deaths (Apoptosis).
Significant mention goes namely towards this rather convoluted evolution process that neoplastic metamorphoses undergo which tend to challenge practitioners making such ailments formidable adversaries demanding complex treatment protocols but also let’s not forget about another aspect i.e., leveraging Warburg effect against carcinogenic substances.
In terms of identifying diseases, mankind has utilized this cancer metabolism occurrence for detection purposes. The test known as positron emission tomography (PET) highlights tissues and organs with unusual metabolic activity.
As cancerous cells exhibit the Warburg effect, they absorb radioactive glucose at a rate hundreds of times greater than standard tissue surrounding them. Consequently, tumors become illuminated in the resulting images allowing easy tracking throughout the entire body.
As far as treatment goes; acknowledging that almost all previous anti-cancer treatments focused solely on gene-centered techniques could position the ‘Warburg’ paradigm shift to potentially be seen as an overlooked “Achilles’ heel” within any approach targeted towards treating cancers over nearly a century’s time span. An alternate compound – Sodium dichloroacetate (DCA) operates uniquely by restoring abnormal cell functions related to metabolism back towards equilibrium
Necessity for Immunotherapy
Cancer cells have developed diverse mechanisms to camouflage themselves rendering them undetectable by our body’s immune defense. They may possess genetic mutations or exhibit distinct surface proteins – both factors can hinder the immune system from discerning and combating these cells.
This is where TILs are an integral part in this scenario. If a patient’s tumors do not consist of these TILs, it signifies that the defensive immune response isn’t effectively battling against the malignancy necessitating the need for immunotherapeutic intervention.
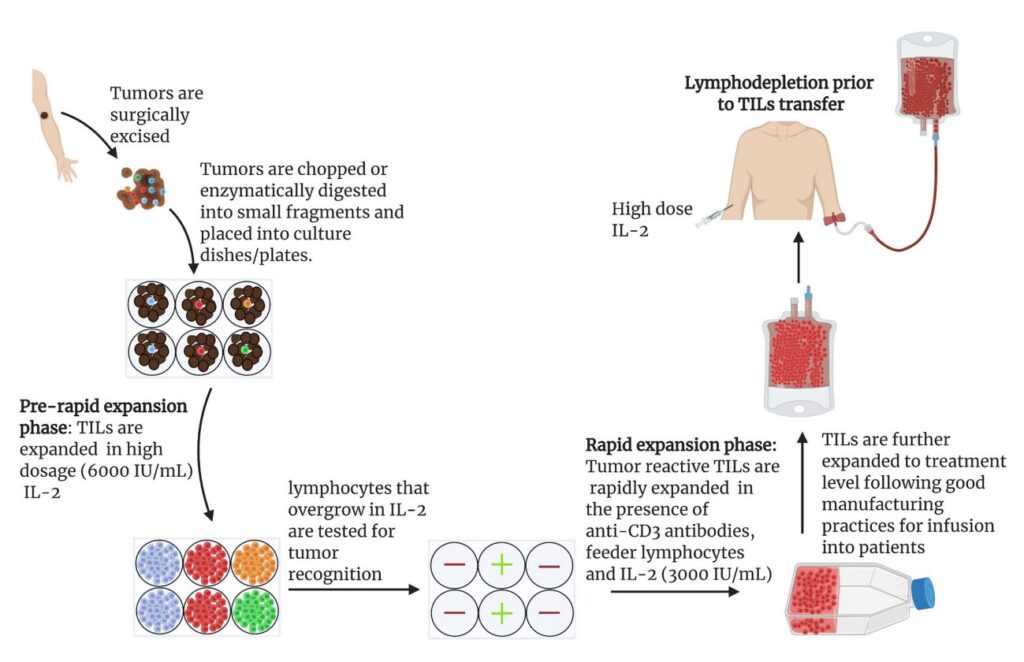
Cancer Immunotherapy Difficulties
Cancer treatment has entered a fresh phase with immunotherapy, bringing optimism to many people. Nonetheless, this approach isn’t without its complications. A main worry is the capacity of cancerous cells to build tolerance over time. Due to their high energy production via glycolysis process, these harmful cells often block specific pathways important in managing T-cells’ functions effectively.
If these T-cells aren’t accurately matured they fail at successfully identifying and neutralizing tumor cells efficiently causing them instead promotion by previous strength gained from the therapeutic antibodies given previously.
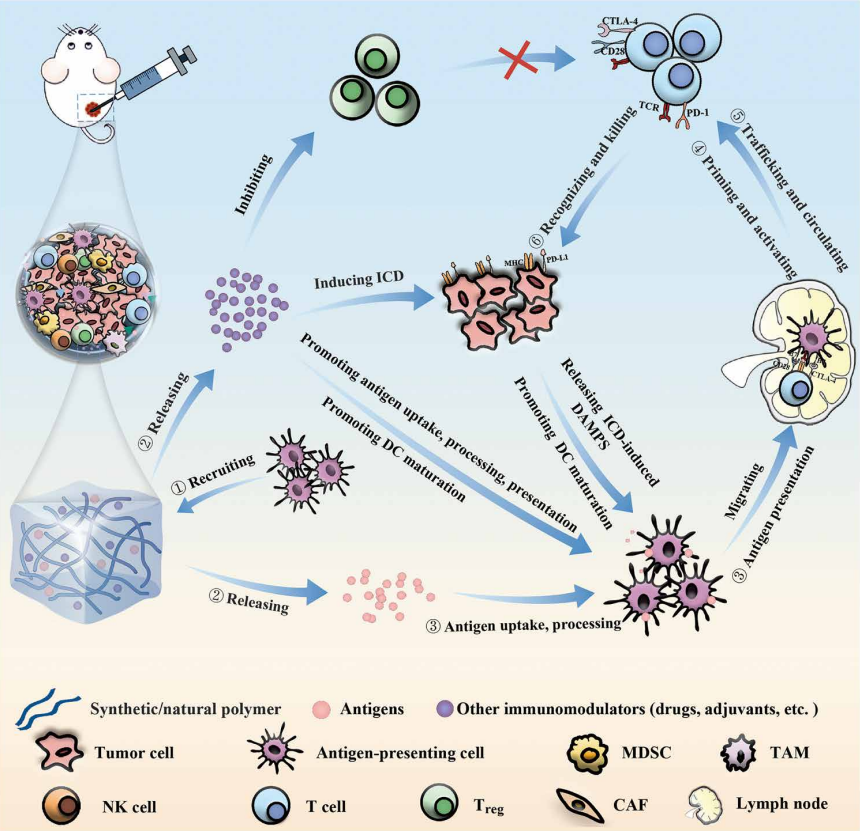
Tackling the Difficulties
In response to these hurdles, directing efforts towards inhibiting the energy production system of tumor cells post-immunotherapy could prove advantageous. By restricting their power supply, there is a potential that they would be incapable of blocking signal paths which dictate T-cell development and control.
Latest Findings in Scientific Studies: 2-deoxy-D-glucose (2DG)
In cancer treatment, especially for Triple Negative Breast Cancer (TNBC), there’s an exciting development.
Scientists have discovered that combining immunotherapy with a glucose antimetabolite known as 2DG could enhance treatment effectiveness. This is particularly noteworthy because TNBC cells often show high levels of a protein called programmed death-ligand 1 (PD-L1).
The thing about treating TNBC: only a small group of patients, roughly 10 to 20%, actually see positive results from therapies that target PD-L1 or PD-1, which are key players in the body’s immune response.
This new approach, involving 2DG, could potentially change the game for many TNBC patients who currently have limited treatment responses.
Here’s a key point: a particular form of PD-L1, known as glycosylated PD-L1, is crucial for the interactions that take place within the PD-L1-PD-1 framework. What’s more, cells associated with TNBC tend to have higher levels of this glycosylated PD-L1 compared to non-TNBC cells.
Research has shown that by significantly impacting the stability and function of this immune-inhibiting pro-cancer molecule, the amount of glycosylation on PD-L1 can be reduced.
This is where “deoxyglucose” comes into play. Using deoxyglucose may potentially disrupt this process, leading to new strategies that combine different approaches for more effective TNBC treatment outcomes.
Cancer Treatment Techniques: Conventional and Progressive Methods
The journey towards efficacious cancer solutions involves numerous methods, spanning from conventional to more avantgarde techniques.
Established treatment procedures such as chemotherapy and radiotherapy are widely acknowledged, while novel elements like 2DG have become progressively attractive due to their prospective advantages in treating cancer either individually or supplemented with well-established aids like fenbendazole and DCA that also contribute significantly in combating the disease.

Chemotherapy
The utilization of chemotherapy is still a pivotal element in combating the disease known as cancer. It’s specifically effective for conditions such as breast cancer, where it’s used to minimize tumor size and facilitate easier surgical removal – an approach referred to preoperative chemotherapy or PCT.
However, even though it may decrease tumor dimensions, we cannot always rely on this method alone because there are numerous instances when the malignant cells reappear post-treatment.
Boosting Chemotherapy's Impact through 2DG
Studies indicate that intertwining chemotherapy with 2DG may fortify its effectiveness. Functionally resembling glucose, 2DG is capable of interfering in the metabolic activities within cancer cells and this could potentially escalate the impact from regular chemotherapy treatments.
The dual utilization symbolizes potential for a comprehensive pathologic response which implies stronger and more prolonged treatment results are achievable. Numerous laboratory tests as well as animal studies have been executed to confirm this combined strategy.
Radiation Therapy
Within cancerous cells, the standalone method to regulate sugar metabolism is 2DG but it also gets fused with therapy forms such as Chemotherapy and Radiotherapy.
Realistically speaking, radiotherapy plays a crucial role when treating cancer. Approximately 60% of those battling this cruel disease will experience this type of treatment either singularly or in conjunction with other treatments.
Radiotherapy, while a powerful tool in the fight against cancer, isn’t without its challenges. It’s effective, no doubt, but it can have some unwelcome side effects over time. One major concern is that cancer cells might develop a resistance to the radiation, and there’s also the risk of damaging healthy cells.
To tackle these issues, especially the problem of radiation tolerance, it’s crucial to bring glycolytic inhibitors into the mix. These inhibitors work by cutting off the energy supply to the cancer cells, essentially starving them of the nutrients they need.
This makes it harder for these cancer cells to repair themselves after being hit by radiation, leading to their eventual breakdown and destruction. Such an approach is a step forward in making radiation therapy safer and more effective for patients.
Radiotherapy Enhancement via 2-Deoxy-D-Glucose
2DG is instrumental as a glycolytic inhibitor. Its function involves binding to the GLUT1 glucose transporter protein before being phosphorylated by Hexokinase enzyme. However, its metabolic progression ends there, ensuring that the glycolytic route stays obstructed.
As such, cancer cells exposed to this inhibitor fail in producing energy due to interrupted normal glucose’s glycolysis process. Besides this, 2DG incites caspase 3 and PARP enzymes’ functions leading up apoptosis within malignant cells while leaving healthy cells unaffected.
In cancer treatment, there’s a promising combination on the horizon: 2-deoxy-D-glucose (2DG) paired with radiotherapy. This duo could significantly boost the power of radiation therapy. How?
Well, 2DG acts as a glucose inhibitor. It interferes with the cancer cells’ ability to repair themselves after being damaged by radiation, mainly because these cells are starved of glucose and essential nutrients.
But 2DG isn’t just a one-trick pony. It serves multiple purposes. Apart from being a disruptor, it also amplifies the impact of radiation treatment. Its action is tied to altering the balance of oxidation-reduction processes within cells.
An important aspect to note is that oxidative stress is believed to be a key pathway through which ionizing radiation kills cancer cells. This new approach, using 2DG in conjunction with radiotherapy, could be a game-changer in effectively treating cancer.
Cancer Cell Diversity
Cancer cells are a diverse bunch, something scientists call tumor heterogeneity, and it’s something you’ll find in almost every type of cancer out there. This diversity often stems from mistakes made during the process of DNA replication when cells are dividing.
Here’s what happens: As cells divide, if there are errors in copying their DNA, new cells with mutations are born. Over time, especially with certain environmental factors acting as triggers, these mutated cells can evolve and potentially lead to cancer. It’s a slow process, but it’s how many cancers begin their journey.
Various Shapes of Cancer Disparity
The differentiation observed in cancer cells, termed as tumor heterogeneity, can be simply divided into two divisions:
Spatial Tumor Heterogeneity
This means that cancer cells don’t spread evenly across the affected tissue. Instead, they tend to spread in a haphazard and unpredictable way.
Timeline Fluctuations in Tumor Diverseness
As tumors develop over time, they often evolve into different subtypes. This process is known as temporal heterogeneity. Essentially, the longer a tumor grows, the more varied it can become.
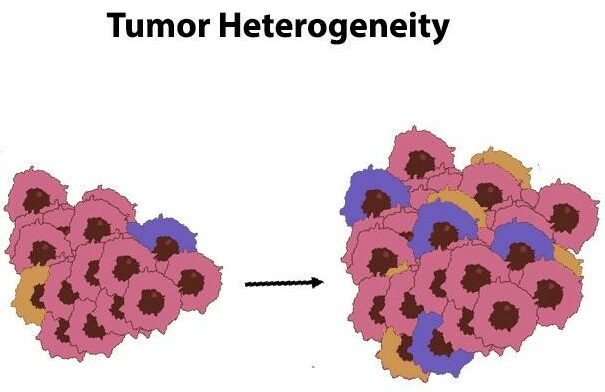
The varying characteristics of tumor cells pose significant obstacles when it comes to combating cancer effectively. The diverse nature of these malignant cells frequently results in resistance towards present therapeutic strategies.
Given this complexity in cancer behavior, there’s a pressing need to develop treatments that can effectively target and overcome the resistance caused by the varied genetic makeup found within individual cancer cells.
Trial Outcomes
A wide range of studies, including lab tests, animal studies, and human clinical trials, have taken a deep dive into the effects of 2-deoxy-D-glucose (2DG) on cells. What’s emerging from this research is that 2DG works in a complex way.
It’s not just stopping the process of cellular glycolysis; it’s also promoting apoptosis, or programmed cell death, without harming healthy cells. Moreover, 2DG impacts how genes behave within cells.
It disrupts transcription factors that are key to activating genes essential for cell replication. This insight points to the potential of 2DG as a weapon against various cancer cell characteristics.
One thing to note about cancer cells is their diverse genetic mutations, which play a role in the progression of the disease. However, they all tend to have one thing in common: a higher rate of sugar metabolism compared to normal cells.
This makes 2DG a potentially effective treatment for a variety of cancers. It works by starving the cancer cells of sugar and oxygen, while also affecting their gene activity. This approach can slow down cell growth and steer them toward self-destruction, preventing excessive growth.
Prospective Cure for Glioblastoma Cancer
Glioblastoma stands out as a particularly aggressive form of brain cancer, known for its strong resistance to cell death and the limited success of current treatments. This situation underscores the urgent need to explore new treatment options.
Not too long ago, Dr. Evangelos Michelakis, a devoted medical scientist, and his team embarked on several research projects focused on Glioblastoma multiforme (GBM) therapy. They realized that an ideal treatment should be able to cross the blood-brain barrier and target cancer cells specifically, without harming healthy tissues.
Their choice, Sodium Dichloroacetate (DCA), displayed these qualities and importantly, showed no signs of causing damage to the blood, heart, kidneys, or liver.
This discovery led them to conduct a landmark study involving real patients, paving the way for further groundbreaking findings in the treatment of GBM.
DCA's Impact on Cancer Cell Demise and Tumor Development
It’s becoming clear that Dichloroacetate (DCA) has a significant impact on fighting cancer, especially in the case of Glioblastoma, a deadly form of brain cancer. DCA has the ability to induce the death of cancer cells, reduce the blood vessels feeding tumors, and slow down the growth of this aggressive cancer.
What’s particularly promising is that DCA might be most effective when used alongside other treatments, offering a new ray of hope in battling this challenging disease.
Alongside Other Therapies for Glioblastoma
In a rare opportunity, scientists got the chance to compare brain tissue from patients before and after they were treated with DCA. What they saw was encouraging: a reduction in cancer cells in the brain tissue after treatment.
This was attributed to an increase in the destruction of deadly cells and a decrease in the growth of malignant glioblastoma multiforme cells. The research concluded that combining DCA with other treatments could be an effective strategy against GBM.
The research team suggests using dichloroacetate, which is known for being non-toxic, either before or after procedures like radiotherapy or surgery. This approach could potentially enhance the benefits of standard treatments for GBM.